Provide Examples of Cell Components Made From Each of the Families of Biochemicals.
General Concepts
Classification
Leaner are classified and identified to distinguish amidst strains and to grouping them by criteria of involvement to microbiologists and other scientists.
Nomenclature
Bacteria are named and so that investigators tin define and discuss them without the necessity of listing their characteristics.
Species
Species, groups of similar organisms within a genus, are designated past biochemical and other phenotypic criteria and by DNA relatedness, which groups strains on the basis of their overall genetic similarity.
Diagnostic Identification
Bacteria are identified routinely by morphological and biochemical tests, supplemented as needed by specialized tests such equally serotyping and antibiotic inhibition patterns. Newer molecular techniques permit species to be identified by their genetic sequences, sometimes direct from the clinical specimen.
Subtyping
Because of differences in pathogenicity or the necessity to narrate a illness outbreak, strains of medical interest are frequently classified below the species level past serotyping, enzyme typing, identification of toxins or other virulence factors, or characterization of plasmids, protein patterns, or nucleic acids.
New and Unusual Species
Laboratories take no difficulty identifying nearly leaner. Problems develop with atypical strains and rare or newly described species; misidentification can lead to inappropriate patient intendance. Therefore, laboratory personnel and physicians (at least communicable diseases specialists) must remain electric current regarding changes in taxonomy and the recognition of new species.
Role of the Clinical Laboratory
Clinical laboratory scientists detect, isolate, identify, and determine the antimicrobial susceptibility patterns of clinically relevant microbes at the asking of physicians, and interface with public health laboratories.
Introduction
Bacteria are classified and identified to distinguish one organism from another and to group similar organisms past criteria of interest to microbiologists or other scientists. The most important level of this type of classification is the species level.
A species name should mean the aforementioned matter to everyone. Within one species, strains and subgroups can differ by the disease they produce, their environmental habitat, and many other characteristics. Formerly, species were created on the basis of such criteria, which may be extremely important for clinical microbiologists and physicians merely which are non a sufficient basis for establishing a species. Verification of existing species and creation of new species should involve biochemical and other phenotypic criteria likewise as Dna relatedness. In numerical or phenetic approaches to classification, strains are grouped on the basis of a large number of phenotypic characteristics. Dna relatedness is used to group strains on the basis of overall genetic similarity.
Species are identified in the clinical laboratory by morphological traits and biochemical tests, some of which are supplemented by serologic assessments (e.g., identification of Salmonella and Shigella species). Considering of differences in pathogenicity (Escherichia coli) or the necessity to characterize a disease outbreak (Vibrio cholerae, methicillin-resistant Staphylococcus aureus), strains of medical involvement are often classified below the species level by serology or identification of toxins. Pathogenic or epidemic strains also can be classified by the presence of a specific plasmid, by their plasmid profile (the number and sizes of plasmids), or by bacteriophage susceptibility patterns (phage typing). Newer molecular biologic techniques have enabled scientists to place some species and strains (without the utilise of biochemical tests) by identifying a specific gene or genetic sequence, sometimes directly from the clinical specimen.
Laboratories accept no difficulty in identifying typical strains of common bacteria using commonly available test systems. Problems do ascend, however, when singular strains or rare or newly described species are non in the data base. Such difficulties are compounded when the strains are misidentified rather than unidentified, and then laboratory personnel and physicians (at least infectious diseases specialists) should be familiar with taxonomic reference texts and journals that publish papers on new species. Bacterial classification at the genus and species level changes frequently, based primarily on the apply of newer genetic techniques. A species may acquire more than one name. In some cases the recognition of a new species results in a unique correlation with specific clinical issues. For case, recognition of Porphyromonas gingivalis as a unique species, divide from its previous inclusion within Bacteroides melaninogenicus (now known to exist equanimous of several taxonomic groups of black-pigmenting anaerobic gram-negative bacilli), elucidated its role as a key pathogen in developed periodontitis. It is important to understand why these changes and synonyms exist in taxonomy.
The clinical laboratory is concerned with the rapid, sensitive, and accurate identification of microbes involved in producing disease. The number and types of tests done in such a laboratory depend on its size and the population it serves. Highly specialized or rarely performed tests should be washed only by reference laboratories. Physicians, clinical laboratory personnel, and reference laboratory personnel must have a expert working relationship if patients are to receive first-charge per unit care.
In add-on, the medico and the clinical laboratory personnel must know which diseases and isolates are reportable to public health laboratories and how to report them .
Definitions
Taxonomy
Taxonomy is the science of classification, identification, and nomenclature. For classification purposes, organisms are usually organized into subspecies, species, genera, families, and higher orders. For eukaryotes, the definition of the species unremarkably stresses the ability of similar organisms to reproduce sexually with the formation of a zygote and to produce fertile offspring. Still, bacteria practise not undergo sexual reproduction in the eukaryotic sense. Other criteria are used for their classification.
Classification
Classification is the orderly organisation of bacteria into groups. There is nothing inherently scientific nearly classification, and different groups of scientists may allocate the same organisms differently. For example, clinical microbiologists are interested in the serotype, antimicrobial resistance pattern, and toxin and invasiveness factors in Escherichia coli, whereas geneticists are concerned with specific mutations and plasmids.
Identification
Identification is the practical use of classification criteria to distinguish sure organisms from others, to verify the actuality or utility of a strain or a detail reaction, or to isolate and identify the organism that causes a disease.
Nomenclature
Nomenclature (naming) is the means by which the characteristics of a species are divers and communicated among microbiologists. A species name should mean the same matter to all microbiologists, withal some definitions vary in different countries or microbiologic specialty groups. For example, the organism known every bit Clostridium perfringens in the United States is called Clostridium welchii in England.
Species
A bacterial species is a singled-out organism with sure characteristic features, or a group of organisms that resemble one some other closely in the near of import features of their system. In the past, unfortunately, there was little agreement nigh these criteria or about the number of features necessary to distinguish a species. Species were oftentimes defined solely by such criteria every bit host range, pathogenicity, or power to produce gas during the fermentation of a given sugar. Without a universal consensus, criteria reflected the interests of the investigators who described a particular species. For case, leaner that caused institute diseases were often divers by the plant from which they were isolated; likewise, each new Salmonella serotype that was discovered was given species condition. These practices accept been replaced by generally accustomed genetic criteria that tin be used to define species in all groups of bacteria.
Approaches to Taxonomy
Numerical Approach
In their studies on members of the family Enterobacteriaceae, Edwards and Ewing established the following principles to characterize, classify, and place organisms (Lennette et al., 1985):
Nomenclature and identification of an organism should be based on its overall morphologic and biochemical pattern. A single characteristic (pathogenicity, host range, or biochemical reaction), regardless of its importance, is non a sufficient basis for classifying or identifying an organism.
A large and diverse strain sample must exist tested to determine accurately the biochemical characteristics used to distinguish a given species.
Singular strains often are perfectly typical members of a given biogroup within an existing species, merely sometimes they are typical members of an unrecognized new species.
In numerical taxonomy (also called computer or phenetic taxonomy) many (l to 200) biochemical, morphological, and cultural characteristics, as well as susceptibilities to antibiotics and inorganic compounds, are used to make up one's mind the caste of similarity between organisms. In numerical studies, investigators often calculate the coefficient of similarity or pct of similarity between strains (where strain indicates a single isolate from a specimen). A dendrogram or a similarity matrix is constructed that joins individual strains into groups and places i group with other groups on the footing of their percentage of similarity. In the dendrogram in Figure 3-1, group 1 represents three Citrobacter freundii strains that are about 95 percent similar and join with a fourth C. freundii strain at the level of ninety percent similarity. Grouping 2 is composed of iii Citrobacter diversus strains that are 95 percent similar, and group 3 contains two E. coli strains that are 95 percent similar, too as a third E. coli strain to which they are 90 percent similar. Similarity betwixt groups ane and 2 occurs at the 70 percent level, and group 3 is most 50 percent similar to groups one and ii.
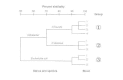
In some cases, certain characteristics may exist weighted more heavily; for example, the presence of spores in Clostridium might be weighted more heavily than the organism's ability to utilise a specific carbon source. A given level of similarity can exist equated with relatedness at the genus, species, and, sometimes, subspecies levels. For instance, strains of a given species may cluster at a 90% similarity level, species within a given genus may cluster at the seventy percent level, and different genera in the same family may cluster at the 50 percent or lower level (Fig. 3-1).
When this approach is the just basis for defining a species, it is difficult to know how many and which tests should be chosen; whether and how the tests should exist weighted; and what level of similarity should be chosen to reflect relatedness at the genus and species levels.
Well-nigh bacteria have enough Dna to specify some ane,500 to vi,000 average-sized genes. Therefore, even a battery of 300 tests would assay only 5 to twenty percent of the genetic potential of a bacterium. Tests that are insufficiently simple to conduct (such as those for saccharide utilization and for enzymes, presence of which can exist assayed colorimetrically) are performed more often than tests for structural, reproductive, and regulatory genes, presence of which is difficult to assay. Thus, major differences may go undetected.
Other types of errors may occur when species are classified solely on the footing of phenotype. For example, different enzymes (specified by unlike genes) may catalyze the same reaction. Besides, even if a metabolic factor is functional, negative reactions can occur because of the disability of the substrate to enter the prison cell, considering of a mutation in a regulatory cistron, or by product of an inactive protein. There is not necessarily a one-to-one correlation between a reaction and the number of genes needed to carry out that reaction. For instance, six enzymatic steps may be involved in a given pathway. If an analysis for the end production is performed, a positive reaction indicates the presence of all half-dozen enzymes, whereas a negative reaction can mean the absence or nonfunction of one to six enzymes. Several other strain characteristics tin can affect phenotypic label; these include growth rate, incubation temperature, salt requirement, and pH. Plasmids that acquit metabolic genes can enable strains to deport out reactions atypical for strains of that species.
The aforementioned prepare of "definitive" reactions cannot be used to classify all groups of organisms, and there is no standard number of specific reactions that allows identification of a species. Organisms are identified on the basis of phenotype, just, from the taxonomic standpoint, definition of species solely on this basis is subject to error.
Phylogenetic Approach
The platonic means of identifying and classifying bacteria would be to compare each gene sequence in a given strain with the gene sequences for every known species. This cannot be done, but the total DNA of i organism can be compared with that of any other organism by a method called nucleic acid hybridization or DNA hybridization. This method can be used to measure out the number of Deoxyribonucleic acid sequences that any two organisms take in common and to judge the percentage of divergence within Dna sequences that are related only non identical. DNA relatedness studies have been done for yeasts, viruses, bacteriophages, and many groups of bacteria.
Five factors can be used to make up one's mind Deoxyribonucleic acid relatedness: genome size, guanine-plus-cytosine (One thousand+C) content, Deoxyribonucleic acid relatedness under weather optimal for DNA reassociation, thermal stability of related DNA sequences, and DNA relatedness under conditions supraoptimal for Dna reassociation. Because information technology is not practical to comport these genotypic or phylogenetic evaluations in clinical laboratories, the results of simpler tests usually must be correlated with known phylogenetic data. For example, yellow strains of Enterobacter cloacae were shown, by Dna relatedness, to class a split up species, Enterobacter sakazakii, merely were non designated equally such until results of practical tests were correlated with the Dna information to allow routine laboratories to place the new species.
Genome Size
Truthful bacterial DNAs have genome sizes (measured equally molecular weight) between 1 × 109 and 8 × 109. Genome size determinations sometimes can distinguish between groups. They were used to distinguish Legionella pneumophila (the legionnaire'south disease bacterium) from Bartonella (Rickettsia) quintana, the agent of trench fever. Fifty. pneumophila has a genome size of nearly 3 × 109; that of B. quintana is almost 1 × 109.
Guanine-plus-Cytosine Content
The G+C content in bacterial DNA ranges from about 25 to 75 percentage. This percentage is specific, but not exclusive, for a species; ii strains with a like G+C content may or may not vest to the same species. If the G+C contents are very dissimilar, however, the strains cannot be members of the aforementioned species.
Dna Relatedness under Atmospheric condition Optimal for Dna Reassociation
DNA relatedness is adamant by allowing single-stranded DNA from 1 strain to reassociate with single-stranded Dna from a 2nd strain, to class a double-stranded DNA molecule (Figure iii-2). This is a specific, temperature-dependent reaction. The optimal temperature for Deoxyribonucleic acid reassociation is 25 to thirty°C below the temperature at which native double-stranded Deoxyribonucleic acid denatures into unmarried strands. Many studies point that a bacterial species is equanimous of strains that are lxx to 100 percent related. In dissimilarity, relatedness between different species is 0 to about 65 percent. It is important to emphasize that the term "related" does not mean "identical" or "homologous." Similar but nonidentical nucleic acid sequences can reassociate.

Figure 3-ii
Diagram of DNA reassociation. DNA is composed of 2 purine nucleoside bases, adenine (A) and guanine (G), and 2 pyrimidine nucleoside bases, thymine (T) and cytosine (C). Double-stranded Dna is formed through hydrogen bonds that can occur only between (more than...)
Thermal Stability of Related DNA Sequences
Each 1 pct of unpaired nucleotide bases in a double-stranded Dna sequence causes a 1 percent decrease in the thermal stability of that Dna duplex. Therefore, a comparison between the thermal stability of a control double-stranded molecule (in which both strands of DNA are from the same organism) and that of a heteroduplex (DNA strands from two different organisms) allows assessment of divergence between related nucleotide sequences.
Dna Relatedness under Supraoptimal Conditions for DNA Reassociation
When the incubation temperature used for DNA reassociation is raised from 25-xxx° C below the denaturation temperature to merely 10-15° C below the denaturation temperature, simply very closely related (and therefore highly thermally stable) Dna sequences can reassociate. Strains from the same species are sixty pct or more than related at these supraoptimal incubation temperatures.
Defining Species on the Basis of Deoxyribonucleic acid Relatedness
Use of these five factors allows a species definition based on DNA. Thus, E. coli tin be defined equally a serial of strains with a Chiliad+C content of 49 to 52 moles percent, a genome molecular weight of 2.3 × 10nine to iii.0 × x9, relatedness of lxx percent or more at an optimal reassociation temperature with 0 to iv percent difference in related sequences, and relatedness of threescore percent or more than at a supraoptimal reassociation temperature. Experience with more than 300 species has produced an arbitrary phylogenetic definition of a species to which most taxonomists subscribe: "strains with approximately 70% or greater DNA-Deoxyribonucleic acid relatedness and with 5° C or less divergence in related sequences." When these two criteria are met, genome size and Grand+C content are ever similar, and relatedness is most e'er 60 percentage or more at supraoptimal incubation temperatures. The 70 percent species relatedness rule has been ignored occasionally when the existing nomenclature is deeply ingrained, every bit is that for E. coli and the iv Shigella species. Because these organisms are all seventy percent or more related, DNA studies point that they should exist grouped into a single species, instead of the present five species in two genera. This change has not been fabricated considering of the presumed confusion that would effect.
DNA relatedness provides one species definition that can exist practical equally to all organisms. Moreover, it cannot be affected past phenotypic variation, mutations, or the presence or absence of metabolic or other plasmids. It measures overall relatedness, and these factors affect only a very small pct of the total DNA.
Polyphasic Approach
In do, the approach to bacterial taxonomy should exist polyphasic (Fig. 3-three). The first step is phenotypic grouping of strains by morphological, biochemical and any other characteristics of interest. The phenotypic groups are then tested for Deoxyribonucleic acid relatedness to determine whether the observed phenotypic homogeneity (or heterogeneity) is reflected by phylogenetic homogeneity or heterogeneity. The third and virtually important step is reexamination of the biochemical characteristics of the Dna relatedness groups. This allows conclusion of the biochemical borders of each grouping and determination of reactions of diagnostic value for the grouping. For identification of a given organism, the importance of specific tests is weighted on the basis of correlation with Dna results. Occasionally, the reactions commonly used will not distinguish completely between two distinct DNA relatedness groups. In these cases, other biochemical tests of diagnostic value must be sought.
Phenotypic Characteristics Useful in Nomenclature and Identification
Morphologic Characteristics
Both wet-mounted and properly stained bacterial jail cell suspensions can yield a great deal of data. These simple tests can indicate the Gram reaction of the organism; whether it is acid-fast; its motion; the arrangement of its flagella; the presence of spores, capsules, and inclusion bodies; and, of course, its shape. This information oftentimes can let identification of an organism to the genus level, or tin can minimize the possibility that it belongs to one or another group. Colony characteristics and pigmentation are also quite helpful. For case, colonies of several Porphyromonas species autofluoresce nether long-wavelength ultraviolet lite, and Proteus species swarm on appropriate media.
Growth Characteristics
A master distinguishing characteristic is whether an organism grows aerobically, anaerobically, facultatively (i.e., in either the presence or absenteeism of oxygen), or microaerobically (i.east., in the presence of a less than atmospheric partial pressure of oxygen). The proper atmospheric conditions are essential for isolating and identifying leaner. Other important growth assessments include the incubation temperature, pH, nutrients required, and resistance to antibiotics. For example, i diarrheal disease agent, Campylobacter jejuni, grows well at 42° C in the presence of several antibiotics; another, Y. enterocolitica, grows better than near other bacteria at 4° C. Legionella, Haemophilus, and some other pathogens require specific growth factors, whereas E. coli and most other Enterobacteriaceae tin can grow on minimal media.
Antigens and Phage Susceptibility
Jail cell wall (O), flagellar (H), and capsular (K) antigens are used to aid in classifying sure organisms at the species level, to serotype strains of medically of import species for epidemiologic purposes, or to identify serotypes of public health importance. Serotyping is also sometimes used to distinguish strains of exceptional virulence or public wellness importance, for example with V. cholerae (O1 is the pandemic strain) and E. coli (enterotoxigenic, enteroinvasive, enterohemorrhagic, and enteropathogenic serotypes).
Phage typing (determining the susceptibility blueprint of an isolate to a fix of specific bacteriophages) has been used primarily equally an aid in epidemiologic surveillance of diseases caused by Staphylococcus aureus, mycobacteria, P, aeruginosa, V. cholerae, and S. typhi. Susceptibility to bacteriocins has also been used equally an epidemiologic strain marking. In nearly cases recently, phage and bacteriocin typing take been supplanted by molecular methods.
Biochemical Characteristics
Most bacteria are identified and classified largely on the basis of their reactions in a series of biochemical tests. Some tests are used routinely for many groups of leaner (oxidase, nitrate reduction, amino acid degrading enzymes, fermentation or utilization of carbohydrates); others are restricted to a unmarried family unit, genus, or species (coagulase test for staphylococci, pyrrolidonyl arylamidase test for Gram-positive cocci).
Both the number of tests needed and the actual tests used for identification vary from one group of organisms to another. Therefore, the lengths to which a laboratory should go in detecting and identifying organisms must be decided in each laboratory on the basis of its function, the type of population information technology serves, and its resources. Clinical laboratories today base of operations the extent of their work on the clinical relevance of an isolate to the particular patient from which it originated, the public health significance of complete identification, and the overall price-benefit analysis of their procedures. For example, the Centers for Disease Control and Prevention (CDC) reference laboratory uses at least 46 tests to identify members of the Enterobacteriaceae, whereas most clinical laboratories, using commercial identification kits or simple rapid tests, place isolates with far fewer criteria.
Classification Below and In a higher place the Species Level
Beneath the Species Level
Especially for epidemiological purposes, clinical microbiologists must distinguish strains with item traits from other strains in the same species. For case, serotype O157:H7 E. coli are identified in stool specimens because of their association with bloody diarrhea and subsequent hemolytic uremic syndrome.
Beneath the species level, strains are designated as groups or types on the footing of common serologic or biochemical reactions, phage or bacteriocin sensitivity, pathogenicity, or other characteristics. Many of these characteristics are already used and accustomed: serotype, phage type, colicin blazon, biotype, bioserotype (a grouping of strains from the same species with common biochemical and serologic characteristics that set up them apart from other members of the species), and pathotype (e.g., toxigenic Clostridium difficile, invasive Due east. coli, and toxigenic Corynebacterium diphtheriae).
Above the Species Level
In addition to species and subspecies designations, clinical microbiologists must be familiar with genera and families. A genus is a group of related species, and a family is a group of related genera.
An ideal genus would exist composed of species with similar phenotypic and phylogenetic characteristics. Some phenotypically homogeneous genera approach this criterion (Citrobacter, Yersinia, and Serratia). More oft, however, the phenotypic similarity is present, but the genetic relatedness is not. Bacillus, Clostridium, and Legionella are examples of accepted phenotypic genera in which genetic relatedness between species is non 50 to 65 percent, but 0 to 65 percentage. When phenotypic and genetic similarity are not both present, phenotypic similarity by and large should exist given priority in establishing genera. Identification practices are simplified by having the most phenotypically similar species in the same genus. The master consideration for a genus is that it contain biochemically similar species that are convenient or important to consider as a group separate from other groups of organisms.
The sequencing of ribosomal RNA (rRNA) genes, which have been highly conserved through evolution, allows phylogenetic comparisons to be fabricated betwixt species whose full DNAs are essentially unrelated. It also allows phylogenetic classification at the genus, family unit, and higher taxonomic levels. The rRNA sequence data are usually not used to designate genera or families unless supported by similarities in phenotypic tests.
Designation of New Species and Nomenclatural Changes
Species are named according to principles and rules of classification set up forth in the Bacteriological Code. Scientific names are taken from Latin or Greek. The right name of a species or college taxon is adamant past iii criteria: valid publication, legitimacy of the name with regard to the rules of classification, and priority of publication (that is, it must exist the get-go validly published proper name for the taxon).
To be published validly, a new species proposal must contain the species name, a clarification of the species, and the designation of a type strain for the species, and the proper name must be published in the International Journal for Systematic Bacteriology (IJSB). Once proposed, a proper name does non go through a formal process to exist accepted officially; in fact, the contrary is true—a validly published name is assumed to exist correct unless and until it is challenged officially. A claiming is initiated by publishing a request for an opinion (to the Judicial Commission of the International Association of Microbiological Societies) in the IJSB. This occurs only in cases in which the validity of a proper name is questioned with respect to compliance with the rules of the Bacteriological Code. A question of classification that is based on scientific data (for example, whether a species, on the footing of its biochemical or genetic characteristics, or both, should be placed in a new genus or an existing genus) is not settled by the Judicial Commission, but by the preference and usage of the scientific community. This is why there are pairs of names such equally Providencia rettgeri/Proteus rettgeri, Moraxella catarrhalis/Branhamella catarrhalis, and Legionella micdadei/Tatlockia micdadei. More than than ane name may thus exist for a single organism. This is not, however, restricted to bacterial nomenclature. Multiple names exist for many antibiotics and other drugs and enzymes.
A number of genera have been divided into additional genera and species have been moved to new or existing genera, such as Arcobacter (new genus for sometime members of Campylobacter) and Burkholderia species (formerly species of Pseudomonas). Two old Campylobacter species (cinaedi and fennelliae) have been moved to the existing genus Helicobacter in some other case.
The all-time source of information for new species proposals and nomenclatural changes is the IJSB. In addition, the Journal of Clinical Microbiology ofttimes publishes descriptions of newly described microorganisms isolated from clinical sources. Information, including biochemical reactions and sources of isolation, about new organisms of clinical importance, illness outbreaks caused past newer species, and reviews of clinical significance of certain organisms may be found in the Annals of Internal Medicine, Periodical of Infectious Diseases, Clinical Microbiology Reviews, and Clinical Infectious Diseases. The data provided in these publications supplement and update Bergey'south Manual of Systematic Bacteriology, the definitive taxonomic reference text.
Assessing Newly Described Bacteria
Since 1974, the number of genera in the family Enterobacteriaceae has increased from 12 to 28 and the number of species from 42 to more than 140, some of which have not yet been named. Similar explosions have occurred in other genera. In 1974, five species were listed in the genus Vibrio and four in Campylobacter; the genus Legionella was unknown. Today, at that place are at least 25 species in Vibrio, 12 Campylobacter species, and more than forty species in Legionella. The total numbers of genera and species continue to increment dramatically.
The clinical significance of the amanuensis of legionnaire's disease was well known long before information technology was isolated, characterized, and classified every bit Legionella pneumophila. In virtually cases, little is known virtually the clinical significance of a new species at the time information technology is commencement described. Assessments of clinical significance begin afterward clinical laboratories prefer the procedures needed to detect and identify the species and accumulate a body of data.
In fact, the detection and even the identification of uncultivatable microbes from unlike environments are now possible using standard molecular methods. The agents of cat scratch disease (Bartonella henselae) and Whipple's disease (Tropheryma whippelii) were elucidated in this manner. Bartonella henselae has since been cultured from several body sites from numerous patients; T. whippelii remains uncultivated.
New species will go on to be described. Many will be able to infect humans and cause illness, specially in those individuals who are immunocompromised, burned, postsurgical, geriatric, and suffering from acquired immunodeficiency syndrome (AIDS). With today's severely immunocompromised patients, frequently the beneficiaries of avant-garde medical interventions, the concept of "pathogen" holds niggling meaning. Any organism is capable of causing disease in such patients under the appropriate weather.
Role of the Clinical Laboratory
Clinical laboratory scientists should be able to isolate, place, and decide the antimicrobial susceptibility design of the vast majority of human being disease agents and so that physicians tin initiate appropriate handling equally soon as possible, and the source and means of transmission of outbreaks can exist ascertained to control the disease and prevent its recurrence. The need to identify clinically relevant microorganisms both rapidly and cost-effectively presents a considerable challenge.
To exist constructive, the professional clinical laboratory staff must interact with the infectious diseases staff. Laboratory scientists should attend infectious disease rounds. They must go on beside of new engineering science, equipment, and classification and should communicate this information to their medical colleagues. They should translate, qualify, or explain laboratory reports. If a bacterial name is changed or a new species reported, the laboratory should provide background information, including a reference.
The clinical laboratory must exist efficient. A concerted endeavor must be made to eliminate or minimize inappropriate and contaminated specimens and the performance of procedures with piffling or no clinical relevance. Standards for the option, collection, and transport of specimens should be developed for both laboratory and nursing procedure manuals and reviewed periodically by a committee composed of medical, nursing, and laboratory staff. Ongoing dialogues and continuous communication with other health intendance workers concerning topics such as specimen collection, test selection, results interpretation, and new engineering science are essential to maintaining high quality microbiological services.
Biochemical and Susceptibility Testing
Most laboratories today employ either commercially available miniaturized biochemical test systems or automated instruments for biochemical tests and for susceptibility testing.
The kits commonly comprise 10 to xx tests. The examination results are converted to numerical biochemical profiles that are identified by using a codebook or a computer. Carbon source utilization systems with upwards to 95 tests are as well bachelor. Most identification takes 4 to 24 hours. Biochemical and enzymatic test systems for which data bases have non been adult are used past some reference laboratories.
Automated instruments tin can be used to place most Gram-negative fermenters, nonfermenters, and Gram-positive bacteria, merely not for anaerobes. Antimicrobial susceptibility testing can be performed for some microorganisms with this equipment, with results expressed as judge minimum inhibitory drug concentrations. Both tasks accept 4 to 24 hours. If semiautomated instruments are used, some manipulation is done manually, and the cultures (in miniature cards or microdilution plates) are incubated outside of the musical instrument. The exam containers are then read rapidly past the instrument, and the results are generated automatically. Instruments are also available for identification of leaner by cell wall fatty acrid profiles generated with gas-liquid chromatography (GLC), analysis of mycolic acids using loftier performance liquid chromatography (HPLC), and by protein-banding patterns generated by polyacrylamide gel electrophoresis (PAGE). Some other instruments designed to speed laboratory diagnosis of bacteria are those that detect (merely exercise not identify) bacteria in claret cultures, usually faster than manual systems considering of continuous monitoring. Also available are many rapid screening systems for detecting one or a series of specific bacteria, including certain streptococci, Due north. meningitidis, salmonellae, Chlamydia trachomatis, and many others. These screening systems are based on fluorescent antibody, agglutination, or other rapid procedures.
It is important to inform physicians as soon equally a presumptive identification of an etiologic agent is obtained so that appropriate therapy can exist initiated as quickly as possible. Gram stain and colony morphology; acrid-fast stains; and spot indole, oxidase, and other rapid enzymatic tests may permit presumptive identification of an isolate within minutes.
Role of the Reference Laboratory
Despite contempo advances, the armamentarium of the clinical laboratory is far from complete. Few laboratories tin can or should conduct the specialized tests that are ofttimes essential to distinguish virulent from avirulent strains. Serotyping is done only for a few species, and phage typing only rarely. Few pathogenicity tests are performed. Not many laboratories can deport comprehensive biochemical tests on strains that cannot be identified readily by commercially available biochemical systems. Fifty-fifty fewer laboratories are equipped to perform plasmid profiles, cistron probes, or DNA hybridization. These and other specialized tests for the serologic or biochemical identification of some exotic bacteria, yeasts, molds, protozoans, and viruses are best washed in regional reference laboratories. Information technology is not cost-effective for smaller laboratories to store and control the quality of reagents and media for tests that are seldom run or quite complex. In addition, it is impossible to maintain proficiency when tests are performed rarely. Sensitive methods for the epidemiologic subtyping of isolates from disease outbreaks, such equally electrophoretic enzyme typing, rRNA fingerprinting, whole-prison cell protein electrophoretic patterns, and restriction endonuclease analysis of whole-jail cell or plasmid DNA, are used merely in reference laboratories and a few large medical centers.
Specific genetic probes are now available commercially for identifying virulence factors and many leaner and viruses. Genetic probes are among the most common methods used for identification of Mycobacterium tuberculosis and K. avium complex in the U.Due south. today. Probes for Neisseria gonorrhoeae and Chlamydia trachomatis are now being used directly on clinical specimens with fantabulous sensitivity and near universal specificity with aforementioned-day results. Mycobacterial probes are also being evaluated for direct specimen testing.
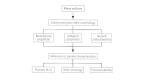
Interfacing with Public Wellness Laboratories
Hospital and local clinical laboratories interact with district, state, and federal public health laboratories in several important means (Fig. 3-4). The clinical laboratories participate in quality control and proficiency testing programs that are conducted by federally regulated agencies. The government reference laboratories supply cultures and frequently reagents for apply in quality control, and they conduct preparation programs for clinical laboratory personnel.

Figure three-four
Pathways for laboratory identification of pathogens and data exchange.
All types of laboratories should interact closely to provide diagnostic services and epidemic surveillance. The primary business organisation of the clinical laboratory is identifying infectious disease agents and studying nosocomial and local outbreaks of disease. When the situation warrants, the local laboratory may ask the country laboratory for assistance in identifying an unusual organism, discovering the cause or way of transmission in a affliction outbreak, or performing specialized tests non washed routinely in clinical laboratories. Cultures should be pure and should exist sent on appropriate media following advisable procedures for transport of biohazardous materials. Pertinent information, including the type of specimen; patient proper noun (or number), date of nativity, and sex; clinical diagnosis, associated illness, date of onset, and present status; specific amanuensis suspected, and any other organisms isolated; relevant epidemiologic and clinical data; treatment of patient; previous laboratory results (biochemical or serologic tests); and necessary data about the submitting political party must accompany each request.
These data allow the country laboratory to test the specimen properly and quickly, and they provide information about occurrences inside the state. For example, a food-borne outbreak might extend to many parts of the state (or across its boundaries). The land laboratory tin can alert local physicians to the possibility of such outbreaks.
Some other necessary interaction between local and state laboratories is the reporting of notifiable diseases by the local laboratory. The state laboratory makes available to local laboratories summaries of the incidence of these diseases. The state laboratories also submit the summaries to the CDC weekly (or, for some diseases, yearly), and national summaries are published weekly in the Morbidity and Mortality Weekly Written report.
Interaction between the CDC and country and federal laboratories is very similar to that between local and land laboratories. The CDC provides quality control cultures and reagents to state laboratories, and serves every bit a national reference laboratory for diagnostic services and epidemiologic surveillance. Local laboratories, however, must initially send specimens to the local or state public health laboratory, which, when necessary, forwards them to the CDC. The CDC reports its results back to the state laboratory, which and so reports to the local laboratory.
Hazards of Clinical Laboratory Work
Clinical laboratory personnel, including support and clerical employees, are subject to the hazard of infection, chemical hazards, and, in some laboratories, radioactive contamination. Such risks can exist prevented or minimized past a laboratory rubber program.
Radiation Hazards
Personnel who work with radioactive materials should have taken a radioactivity safe course; they should wear radiation monitor badges and be aware of the methods for decontaminating easily, clothing, work surfaces, and equipment. They should wear gloves when working with radioactive compounds. When they piece of work with high-level radiation, they should use a hood and stand backside a radiation shield. Preparative radioactive piece of work should be done in a split room with access merely by personnel who are involved directly in the work.
Chemical Hazards
Chemicals can impairment laboratory personnel through inhalation or skin absorption of volatile compounds; bodily contact with carcinogens, acids, bases, and other harmful chemicals; or introduction of poisonous or skin-dissentious liquids into the mouth. Skilful laboratory practices crave that volatile compounds be handled simply nether a hood, that hazardous chemicals never be pipetted by mouth, and that anyone working with skin-damaging chemicals wear gloves, center guards, and other personal protective equipment every bit necessary. Workers should be familiar with the materials safe data sheets (MSDS) posted in an accessible place in every laboratory. These forms incorporate information about chemical hazards and procedures for decontamination should an accident occur.
Biologic Hazards
Microbiologic contamination is the greatest risk in clinical microbiology laboratories. Laboratory infections are a danger non only to the clinical laboratory personnel just besides to anyone else who enters the laboratory, including janitors, clerical and maintenance personnel, and visitors. The adventure of infection is governed by the frequency and length of contact with the infectious agent, its virulence, the dose and route of administration, and the susceptibility of the host. The inherent hazard of whatever infectious agent is affected past factors such as the volume of infectious material used, handling of the material, effectiveness of safety containment equipment, and soundness of laboratory methods. Body fluids from patients, particularly those containing blood, are considered potentially infectious for blood-borne pathogens, and must be handled appropriately.
If possible, agents that are treated differently, such as viruses as opposed to bacteria, or K. tuberculosis in contrast to Due east. coli, should be handled in different laboratories or in unlike parts of the same laboratory. When the take chances category of an agent is known, information technology should exist handled in an surface area with advisable containment. All specimens sent for microbiological studies and all organisms sent to the laboratory for identification should be assumed to be potentially infectious. A separate area should be prepare aside for the receipt of specimens. Personnel should be aware of the potential hazards of improperly packed, broken, or leaking packages and of the proper methods for their handling and decontamination .
To forestall infection, personnel should wearable moisture-proof laboratory coats at all times, wash their easily before and after wearing gloves and at the conclusion of each potential exposure to etiologic agents, refrain from mouth pipetting, and not eat, drink, smoke, or apply cosmetics in the laboratory. Immunization may be advisable for employees who are exposed often to certain infectious agents, including hepatitis B, yellow fever, rabies, polioviruses, meningococci, Y. pestis, S. typhi, and Francisella tularensis. Universal precautions, body substance isolation, and other mandated practices involve the employ of personal protective equipment and engineering controls to minimize laboratory scientists' exposure to claret-borne pathogens, even when the adventure of infection is unknown.
Biosafety Levels
Infectious agents are assigned to a biosafety level from 1 to 4 on the basis of their virulence. The containment levels for organisms should correlate with the biosafety level assigned. Biosafety level 1 is for well-defined organisms non known to cause disease in good for you humans; information technology includes certain nonvirulent East. coli strains (such as 1000-12) and B. subtilis. Containment level 1 involves standard microbiologic practices, and safety equipment is non needed.
Biosafety level 2, the minimum level for clinical laboratories, is for moderate-run a risk agents associated with man affliction. Containment level ii includes limited access to the piece of work expanse, decontamination of all infectious wastes, use of protective gloves, and a biologic safety cabinet for use in procedures that may create aerosols. Examples of biosafety level 2 agents include nematode, protozoan, trematode, and cestode homo parasites; all human fungal pathogens except Coccidioides immitis; all members of the Enterobacteriaceae except Y. pestis; Bacillus anthracis; Clostridium tetani; Corynebacterium diphtheriae; Haemophilus species; leptospires; legionellae; mycobacteria other than Thousand. tuberculosis; pathogenic Neisseria species; staphylococci, streptococci, Treponema pallidum; 5. cholerae; and hepatitis and influenza viruses. Clinical specimens potentially containing some biosafety level 3 agents, such equally Brucella spp., are ordinarily handled using biosafety level 2 containment practices.
Biosafety level three is for agents that are associated with risk of serious or fatal droplets infection. In containment level three, laboratory access is controlled, special vesture is worn in the laboratory, and containment equipment is used for all piece of work with the agent. K. tuberculosis, Coccidioides immitis, Coxiella burnetii, and many of the arboviruses are biosafety 3 level agents. Containment level 3 usually is recommended for work with cultures of rickettsiae, brucellae, Y. pestis, and a wide variety of viruses, including human being immunodeficiency viruses.
Biosafety level 4 indicates dangerous and novel agents that cause diseases with loftier fatality rates. Maximum containment and decontamination procedures are used in containment level 4, which is constitute in merely a few reference and research laboratories. Only a few viruses (including Lassa, Ebola, and Marburg viruses) are classified in biosafety level 4.
References
-
Bergey's Manual of Systematic Bacteriology. Vol. one-4. Williams & Wilkins, Baltimore, 1984-1989.
-
Center for Infectious Diseases. Reference/Diagnostic Services. Centers for Disease Control and Prevention, Atlanta, GA.
-
Centers for Disease Control and Prevention: Morbidity and Mortality Weekly Report.Massachusetts Medical Guild, Waltham, MA.
-
Fleming Practise, Richardson JH, Tulis JI, Vesley D (eds): Laboratory Safety: Principles and Practices. second Ed. ASM Press, Washington, D.C., 1995.
-
Lennette EH, et al (eds): Manual of Clinical Microbiology quaternary Ed. American Society for Microbiology, Washington, D.C., 1985.
-
Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH (eds): Manual of Clinical Microbiology 6th Ed. ASM Printing, Washington, D.C., 1995.
-
Richmond JY, McKinney RW (eds): Biosafety in Microbiological and Biomedical Laboratories. tertiary Ed. Centers for Disease Control and Prevention, Atlanta, GA, and National Institutes of Health, Bethesda, MD, 1993.
-
Skerman VBD, McGowan 5, Sneath PHA (eds): Canonical lists of bacterial names. Int J Syst Bacteriol 30: 225, 1980. [PubMed: 20806452]
-
Sneath, PHA (ed): International Code of Nomenclature of Bacteria: Bacteriological Code, 1990 Revision. American Guild for Microbiology, Washington, D.C., 1990 . [PubMed: 21089234]
Source: https://www.ncbi.nlm.nih.gov/books/NBK8406/
0 Response to "Provide Examples of Cell Components Made From Each of the Families of Biochemicals."
Publicar un comentario